
Engage Introduce students to the idea that electrons surround the nucleus of an atom in regions called energy levels.This is an online version of the periodic table card game from this lesson that you can assign as class work or homework after students have played the game in the classroom. This lesson is intended as a follow-up to chapter 4, lesson 2.įor Lesson 4.3, students can play the Periodic Table Game, Game #2.

You will need the five cards on the right hand side of each sheet. About this Lessonīe sure that the 20 atom name cards are posted around the room. The activity sheet will serve as the “Evaluate” component of each 5-E lesson plan. Evaluationĭownload the student activity sheet, and distribute one per student when specified in the activity. Students will be able to interpret the information given in the periodic table to describe the arrangement of electrons on the energy levels around an atom. They will again try to correctly match the cards with each element.
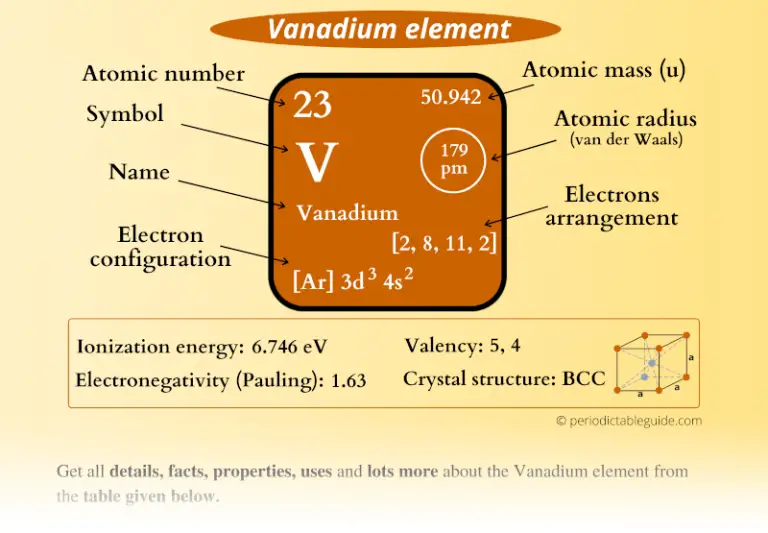
Students will be given cards with information about the electrons and energy levels for each of the first 20 atoms. Students will first look at a diagram and animation to understand the basic pattern of the arrangement of electrons on energy levels around an atom. Students will again focus on the first 20 elements. Atoms in the same column (group) in the periodic table have the same number of valence electrons.The electrons in the energy level farthest from the nucleus are called valence electrons.When the third energy level has 8 electrons, the next 2 electrons go into the fourth energy level.When the second energy level has 8 electrons, the next electrons go into the third energy level until the third level has 8 electrons.When the first energy level has 2 electrons, the next electrons go into the second energy level until the second level has 8 electrons.

Each energy level can accommodate or “hold” a different number of electrons before additional electrons begin to go into the next level.The third is a little farther away than the second, and so on. The second energy level is a little farther away than the first. The first energy level is closest to the nucleus.An energy level represents the 3-dimensional space surrounding the nucleus where electrons are most likely to be.The electrons surrounding an atom are located in regions around the nucleus called “energy levels”.


 0 kommentar(er)
0 kommentar(er)
